Cystathionine-γ-lyase expression is associated with mitochondrial respiration during sepsis-induced acute kidney injury in swine with atherosclerosis
- Merz, Tamara Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany
- Wepler, Martin Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany - Klinik für Anästhesiologie, Universitätsklinikum Ulm, Ulm, Germany
- Nußbaum, Benedikt Klinik für Anästhesiologie, Universitätsklinikum Ulm, Ulm, Germany
- Vogt, Josef Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany
- Calzia, Enrico Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany
- Wang, Rui Department of Biology, Laurentian University, Sudbury, Canada
- Szabo, Csaba Department of Anesthesiology, The University of Texas Medical Branch at Galveston, USA - Department of Oncology, Microbiology and Immunology, Faculty of Science and Medicine, University of Fribourg, Switzerland.
- Radermacher, Peter Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany
- McCook, Oscar Institut für Anästhesiologische Pathophysiologie und Verfahrensentwicklung, Universitätsklinikum Ulm, Germany
-
20.10.2018
Published in:
- Intensive Care Medicine Experimental. - 2018, vol. 6, no. 1, p. 43
English
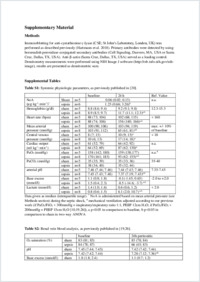
Sepsis is associated with disturbed glucose metabolism and reduced mitochondrial activity and biogenesis, ultimately leading to multiple organ dysfunction, e.g., acute kidney injury (AKI). Cystathionine-γ-lyase (CSE), the major cardiovascular source of endogenous H2S release, is implicated in the regulation of glucose metabolism and mitochondrial activity through a PGC1α-dependent mechanism, and critical for kidney function. Atherosclerosis is associated with mitochondrial dysfunction and reduced CSE expression. Thus, the aim of this post hoc study was to test the hypothesis whether there is an interplay between CSE expression and kidney dysfunction, mitochondrial activity, and oxidative/nitrosative stress in porcine septic AKI with underlying coronary artery disease.Methods: This study is a post hoc analysis of material from anesthetized and instrumented swine with a high fat diet-induced hypercholesterolemia and atherosclerosis undergoing faecal peritonitis-induced septic shock or sham procedure and intensive care (comprising fluid resuscitation and continuous i.v. noradrenaline (NoA) infusion) for 24 h. Glucose metabolism was quantified from blood 13C6-glucose and expiratory 13CO2/12CO2 isotope enrichment during 13C6-glucose infusion. Mitochondrial activity was determined by high- resolution respirometry. CSE and PGC1α expression, as well as nitrotyrosine formation and albumin extravasation, were quantified by immunohistochemistry of formalin-fixed kidney paraffin sections.Results: Sepsis was associated with lactic acidosis (p = 0.004) and AKI (50% fall of creatinine clearance (CrCl), p = 0.019). While both whole-body glucose production (p = 0.004) and oxidation (p = 0.006) were increased, kidney tissue mitochondrial respiration was reduced (p = 0.028), coinciding with decreased CSE (p = 0.003) and PGC1α (p = 0.003) expression. Albumin extravasation (p = 0.011) and nitrotyrosine formation (p = 0.008) were increased in septic kidneys.Conclusions: Sepsis-induced AKI is associated with disturbed mitochondrial respiration and biogenesis, which may be aggravated by oxidative and nitrosative stress. Our results confirm previous data in murine septic shock and porcine hemorrhage and resuscitation on the crucial role of CSE for barrier integrity and kidney function
- Faculty
- Faculté des sciences et de médecine
- Department
- Médecine 3ème année
- Language
-
- English
- Classification
- Biological sciences
- License
- License undefined
- Identifiers
-
- RERO DOC 323469
- DOI 10.1186/s40635-018-0208-z
- Persistent URL
- https://folia.unifr.ch/unifr/documents/307479
Other files
Statistics
Document views: 75
File downloads:
- sza_cea.pdf: 152
- sza_cea_sm.pdf: 93