Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma
- Roehnisch, Tim Division of Hematology and Oncology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
- Then, Cornelia Division of Endocrinology and Diabetology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
- Nagel, Wolfgang Helmholtz Zentrum München, Deutsches Forschungszentrum für Gesundheit und Umwelt, Munich, Germany
- Blumenthal, Christina Division of Hematology and Oncology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
- Braciak, Todd Division of Hematology and Oncology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
- Donzeau, Mariel Maître de conférences (MCF) - Université de Strasbourg, Unité UMR Biotechnologie et Signalisation Cellulaire, Strasbourg, France
- Böhm, Thomas Division of Hematology and Oncology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
- Flaig, Michael Dermatologische Klinik der Universität München, Germany
- Bourquin, Carole Université de Fribourg, Département de Médecine, Chair of Pharmacology, Switzerland
- Oduncu, Fuat S Division of Hematology and Oncology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Germany
-
09.05.2014
Published in:
- Journal of Translational Medicine. - 2014, vol. 12, no. 1, p. 119
Patient specific immunotherapy
Phage idiotype vaccination
Multiple myeloma
Phase I II clinical trial
Paraprotein
English
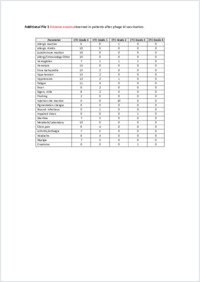
Background: Multiple myeloma is characterized by clonal expansion of B cells producing monoclonal immunoglobulins or fragments thereof, which can be detected in the serum and/or urine and are ideal target antigens for patient-specific immunotherapies.Methods: Using phage particles as immunological carriers, we employed a novel chemically linked idiotype vaccine in a clinical phase I/II trial including 15 patients with advanced multiple myeloma. Vaccines composed of purified paraproteins linked to phage were manufactured successfully for each patient. Patients received six intradermal immunizations with phage idiotype vaccines in three different dose groups.Results: Phage idiotype was well tolerated by all study participants. A subset of patients (80% in the middle dose group) displayed a clinical response indicated by decrease or stabilization of paraprotein levels. Patients exhibiting a clinical response to phage vaccines also raised idiotype-specific immunoglobulins. Induction of a cellular immune response was demonstrated by a cytotoxicity assay and delayed type hypersensitivity tests.Conclusion: We present a simple, time- and cost-efficient phage idiotype vaccination strategy, which represents a safe and feasible patient-specific therapy for patients with advanced multiple myeloma and produced promising anti-tumor activity in a subset of patients.
- Faculty
- Faculté des sciences et de médecine
- Department
- Département de Médecine
- Language
-
- English
- Classification
- Biological sciences
- License
-
License undefined
- Identifiers
-
- RERO DOC 211455
- DOI 10.1186/1479-5876-12-119
- Persistent URL
- https://folia.unifr.ch/unifr/documents/303828
Other files
Statistics
Document views: 153
File downloads:
- pdf: 146
- Supplementary material: 122