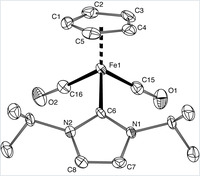
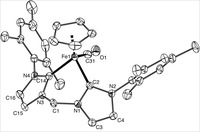
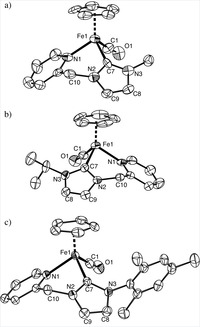
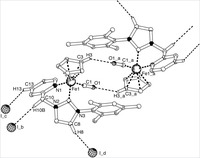
Piano-stool iron(ii) complexes as probes for the bonding of n-heterocyclic carbenes: indications for -acceptor ability
- Mercs, Laszlo Department of Chemistry, University of Fribourg, Switzerland
- Labat, Gaël Institute of Microtechnology, University of Neuchâtel, Switzerland
- Neels, Antonia Institute of Microtechnology, University of Neuchâtel, Switzerland
- Ehlers, Andreas Scheikundig Laboratorium, Vrije Universiteit van Amsterdam, The Netherlands
- Albrecht, Martin Department of Chemistry, University of Fribourg, Switzerland
-
12.10.2006
Published in:
- Organometallics. - 2006, vol. 25, no. 23, p. 5648 -5656
English
A series of new piano-stool iron(II) complexes comprising mono- and bidentate chelating N-heterocyclic carbene ligands [Fe(cp)(CO)(NHC)(L)]X have been prepared and analyzed by spectroscopic, electrochemical, crystallographic, and theoretical methods. Selectively substituting the L site with a series of ligands going from carbene to pyridine to CO suggests that CO is the strongest π acceptor, while the behavior of pyridine and carbene is nearly identical. This suggests that in these complexes comprising an electron-rich iron(cp)(carbene) fragment, N-heterocyclic carbenes are not pure σ donors but also moderate π acceptors. Theoretical calculations support this bonding model and indicate charge saturation at the metal as key for π back-bonding to N-heterocyclic carbenes. On the basis of voltammetric measurements, the Lever electrochemical parameter of these carbenes has been determined: EL = +0.29. Systematic substitution of the wingtip groups of the carbene revealed only subtle changes in the electronic properties of the iron center, thus providing a suitable methodology for ligand-induced fine-tuning of the coordinated metal.
- Faculty
- Faculté des sciences et de médecine
- Department
- Département de Chimie
- Language
-
- English
- Classification
- Chemistry
- License
- License undefined
- Identifiers
-
- RERO DOC 10550
- DOI 10.1021/om060637c
- Persistent URL
- https://folia.unifr.ch/unifr/documents/300797
Other files
Statistics
Document views: 78
File downloads:
- albrecht_psi.pdf: 191
- albrecht_psi_sm.pdf: 182
- albrecht_psi_sm1.tif: 16
- albrecht_psi_sm2.tif: 19
- albrecht_psi_sm3.tif: 13
- albrecht_psi_sm4.tif: 21
- albrecht_psi_sm5.tif: 29
- albrecht_psi_sm6.tif: 13
- albrecht_psi_sm3.cif: 46